The nitrogen cycle, explained
Nitrogen is one of the most essential nutrients for plant growth. Although nitrogen gas in the atmosphere represents the largest quantity of the nutrient in the environment, it’s unavailable to most plants as a direct source. To better understand nutrient and fertilizer management, it’s important to understand the factors that affect nitrogen within the nitrogen cycle.
Three factors that affect nitrogen within the nitrogen cycle
Inputs to the soil
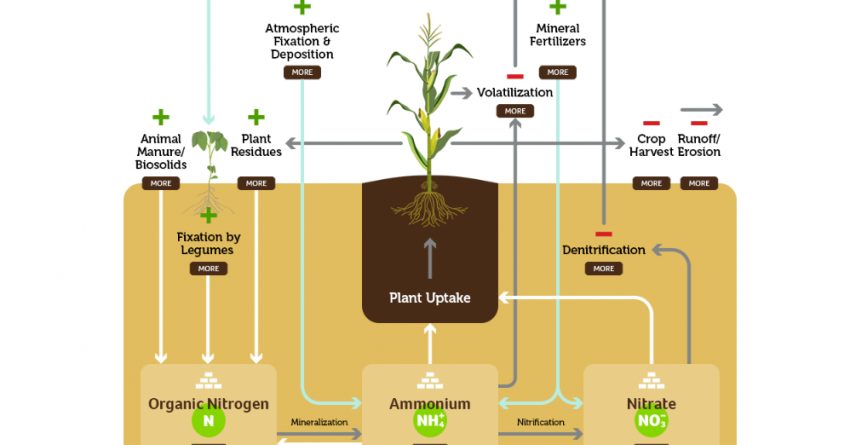
More than 90 per cent of soil nitrogen is found in soil organic matter (animal manure, plant residues, fixation by legumes) in forms unavailable to plants. Organic nitrogen becomes available when soil organic matter is decomposed by soil organisms.
Most of the nitrogen in commercially available fertilizer is derived by combining atmospheric N2 with H2 to form ammonia (NH3), which can be used as fertilizer (anhydrous ammonia) or further processed into other dry or liquid nitrogen fertilizers such as urea, ammonium sulfate or polymer coated nitrogen fertilizers such as ESN.
Losses from the soil
The most common nitrogen fertilizer loss is by the removal of crop portions containing nitrogen during harvest, but losses can also occur by leaching, denitrification, volatilization, or as a result of runoff and erosion. By understanding these forms of nitrogen loss, growers can utilize farming practices, like 4R nutrient stewardship, to minimize the loss of nutrients and ROI.
Crop harvest: This represents the amount of nitrogen that’s in the harvested portion of the crop and removed from the field completely.
Leaching: The nitrate form of nitrogen (NO3) is very soluble and leaches easily when excess water moves through the soil.
Denitrification: When finer-textured soil becomes saturated, some organisms look for oxygen by decomposing NO3 – a process called denitrification. The NO3is converted to gases that are unavailable to plants, escape from the soil, and can cause significant losses of nitrogen when soil is warm and remains saturated for even short periods.
Volatilization: This happens when ammonia gas (NH3) is lost to the atmosphere from anhydrous ammonia, urea, or liquid nitrogen fertilizer sources.
Runoff/erosion: Water that doesn’t infiltrate the soil and is lost by surface flow is runoff. Erosion refers to the erosion of soil particles that are carried away by runoff from rain or irrigation, by wind, and by ice.
Transformation within the soil
Plants can take up two forms of nitrogen: nitrate (NO3-) and ammonium (NH4+). Although you can apply either organic or inorganic forms of nitrogen, plants will only take up these two forms. Once in the soil, all forms of nitrogen undergo chemical changes to ultimately transform into plant-available nitrogen. The three main forms of nutrient transformation within the soil are:
Mineralization: Soil microbes transform organic nitrogen forms in plant residues or organic soil amendments into a plant-available form of nitrogen – ammonium. More than 90 per cent of soil nitrogen is found in soil organic matter in forms unavailable to plants. Organic nitrogen becomes available when soil organic matter is decomposed by soil organisms.
Immobilization: As organisms decompose plant and other organic materials, they utilize available nitrogen in the soil, in the process converting it into organic nitrogen compounds. Immobilization causes a temporary reduction of plant-available nitrogen, but this nitrogen becomes available again, once organisms further decompose these organic compounds. Immobilization is the opposite of mineralization.
Nitrification: Nitrate nitrogen is the form taken up by plants in the largest quantity. In warm, moist soils, ammonium rapidly converts to nitrate by soil microbes to be better absorbed by plant roots. The nitrate form is susceptible to both leaching and denitrification losses.
To learn more about the process and see how each of these factors interact within the nitrogen cycle, visit Nutrien eKonomics for an interactive diagram.